Service & Support
Frequently Asked Questions
The most important difference between the CHIRALPAK immobilised columns and the traditional polysaccharide columns is their robustness and stability to mobile phase composition. In the CHIRALPAK IA, IB, IC, ID, IE, IF and IG chiral columns, the stationary phase is immobilised on the packing material instead of the coating process used in Daicel’s other chiral columns.
This immobilisation confers two major advantages. One is that the Chiral Stationary Phase (CSP) can no longer be changed or destroyed by the use of a “forbidden” solvent — there are no forbidden organic solvents with the new columns. The other advantage is that with this total freedom of choice of solvent it is possible to develop new separations not previously possible, thus further extending the range of polysaccharide-based columns to chiral separations. See Question 8 for information on how the extended range of solvents can be used to attain high levels of success for method development with these new columns.
Some of the chiral selectors of this new generation of columns are also present in the traditional coated series. CHIRALPAK IA, IB and IF are based on the same chiral selectors as CHIRALPAK AD-H, CHIRALCEL OD-H and CHIRALPAK AZ-H, respectively. CHIRALPAK IC, ID, IE and IG bear totally different selectors with no equivalent in the coated series. To view structures of the chiral selectors, click here.
CHIRALPAK IA, IB, IC, ID, IE, IF and IG chiral columns are generally complementary and offer unique chiral separation capabilities. Each column will separate a different set of enantiomeric compounds, although some compounds will resolve on more than one column. Method development strategies have been developed with them in different chromatographic modes and will be discussed accordingly in next sections.
There are currently no known organic solvents which will damage the CHIRALPAK immobilised columns in any way.
Extensive testing has been carried out with many common organic solvents, including hexane or heptane/alcohols, methanol, isopropanol, ethanol, acetonitrile, dichloromethane, chloroform, tetrahydrofuran, ethyl acetate, acetone, methyl acetate, MTBE, dimethylformamide, dimethylacetamide, etc.
See question 7 for the most useful solvent mixtures to be used as mobile phases.
Where the columns have been extensively used and have perhaps become fouled with impurities or non-eluted compounds they can readily be cleaned by flushing with dimethylformamide, THF, or ethyl acetate. Regeneration protocols are detailed in the instruction manuals.
When used in reversed-phase chromatography, the columns should not be operated below pH 2 or above pH 7. The upper range only can be extended to pH 9, provided that a suitable buffer is employed, and that a guard cartridge is used and is changed at least once every 200 injections at this pH.
For analytical purposes, high sample concentrations are usually not necessary. A sample preparation of 1 mg/ml, or even less, in mobile phase is usually sufficient. If your sample is an acid salt of a base, then addition of 0.1% DEA to the sample solvent may help solubility by converting the material to the free base, which usually is more soluble. Conversely, if your sample is a salt of an acid, then addition of 0.1% trifluoroacetic acid may improve solubility.
For optimal method robustness, it is always preferred having the sample dissolved in the mobile phase used to resolved the target molecules. Nevertheless, when this is not possible, there are several options.
If you are using one of the immobilised phases, try to dissolve your sample in some other solvent in which it has good solubility. When the application is analytical, there should be no issues with sample solvent-generated effects on the results, providing the sample injection volume is small. This is especially true if the chosen solvent has a similar solvent strength to that of the mobile phase. For preparative applications, this can be more problematic since the sample can precipitate from solution, once the injection mixes with the mobile phase. In such cases, it is best to use the sample solvent as one of the mobile phase components to enhance the mobile phase solubility.
Where the column used is a traditional, coated column, you may try to dissolve the sample in 100% methanol, ethanol, isopropanol, or acetonitrile. These polar solvents can be used with nearly all of our most popular chiral stationary phases. Mixtures of acetonitrile and alkane must be strictly avoided as they are not miscible and will destroy those columns. In general, organic solvents such as toluene, chloroform, methylene chloride, MTBE, tetrahydrofuran, acetone, MEK, ethyl acetate, dimethylformamide, dimethyl sulfoxide, and pyridine must be avoided when dissolving samples to be injected into the traditional coated columns. Even small amounts of these solvents, not removed during sample clean-up, can dissolve the chiral stationary phase and lead to dramatically shortened column life.
If your sample will only dissolve in aqueous solvents, than it can be resolved in reverse-phase mode with IA, IB, IB N, IC, ID, IE, IF, IG, IH, and IJ columns and RP coated columns. Mobile phases and dilution solvents of methanol/H2O ethanol/H2O, isopropanol/H2O, or acetonitrile/H2O can be used in reverse-phase mode. Please consult the Instruction Sheet for your specific column for reverse-phase composition limitations associated with specific CSPs. Care should be taken to control the pH between 2 and 9 in order to avoid dissolving the silica support of the chiral stationary phase.
Generally, the best procedure for chiral separation is to dissolve a sample in mobile phase, whenever possible. Care must still be taken when injecting sample dissolved in a solvent that has greater solvating power than mobile phase. This is a general chromatographic problem in that this may cause distortion of the chromatographic peaks, thus affecting the analytical results. In preparative chromatography there is a risk that sample from a concentrated injection in a good solvent will precipitate on the column, once the sample comes in contact with a mobile phase in which it has poorer solubility. An additional problem could occur if the column has greater attraction for sample diluent than for mobile phase. In such cases, sample diluent may stick to the column and affect the selectivity or efficiency of future injections.
For some applications, samples are presented in dichloromethane (DCM) or DCM mixtures. Other solvents, such as DMSO or DMF, are also allowed for sample injection but they may produce more perturbance in the front end separation. Precipitation issues may also be encountered.
Of course, since the stationary phase is immobilised, there are no issues with stability of the column under any of the above circumstances. However, frit clogging could be an issue.
Choosing the appropriate chiral column depends on your specific chiral separation needs. Our show chiral recognition abilities in organic and aqueous mixtures in LC, as well as under supercritical fluid (SFC) conditions. Their enantioselectivity, retention and resolution degree is differently modulated in those modes, due to the fact that solvent eluting strength, solvation, polymer swelling/shrinking, interaction forces (hydrogen bonding, π- π interaction, …), etc. will not be equivalent in all of them. Therefore, the choice of the mode will be an essential element in the experimental design.
The standard screening protocols adopted by companies and research groups worldwide usually use three or four analytical columns in the primary screening. Two main options for setting up the primary screening include three to four immobilised phases of newest generation (CHIRALPAK IA, IB, IC and IG) or alternatively the four traditional coated columns (CHIRALPAK AD and AS, CHIRALCEL OD and OJ). Such configurations with polysaccharide derived columns, tested on a randomly composed sample pool, and with four solvent mixtures, routinely afford complete enantiomer resolution for over 90% of the racemates examined and enantiorecognition above 95%.
Those columns constitute the primary screening kits, but a full range of other columns are also available to extend the application domain and undertake the resolution of more complex sample mixtures, other than racemates (isomeric mixtures, impurities, etc).
Three main areas for method development can be considered. To view guidelines on method development strategies, click here.
- Use of organic solvent mixtures in HPLC
- Use of water compatible mixtures in HPLC
- SFC conditions
Chiral Technologies has introduced the Column Selection Service. For primary screening, our experienced technical team will test your material on our Chiral Stationary Phases. Compounds with more than one chiral center are expected to require significantly more effort, and therefore a more extensive screening and optimization regimen will be employed.
If you would like technical assistance in performing a literature search for your application, please contact us, depending on your region: Chiral Technologies Europe (CTE), support@cte.daicel.com, Chiral Technologies, Inc. (CTI), questions@cti.daicel.com,or Daicel Chiral Technologies India (DCTI), chiral@chiral.daicel.com
Please provide structures of the compounds you wish to separate. You can also send names of well-known compounds with similar structural features or specific derivatives of these compounds so that a structure-based search can be performed. In many cases, a separation of your compound using one or more Daicel chiral columns, or one closely related to it, will already have been reported in the literature. Based upon this search, we can usually recommend the column most likely to separate your compound.
More specific guidelines can be found in the DAICEL Application Search for Chiral Column Selection. It lists over 1,100 separations for non-proprietary racemic compounds that have been successfully resolved. These guidelines are based on empirical observations and may not correctly predict the separation of your compound.
Unless there are special circumstances, it is strongly recommended that immobilised columns be used for any application. This is because of their far greater stability in operation than other Daicel chiral columns. Another advantage of immobilised columns is their stability to strong solvents like THF, ethyl acetate, and the chlorinated solvents. This allows development of separations in these solvents which often give different selectivity in comparison with the usual solvent set used for chiral chromatography. Further, in preparative chromatographic applications, the use of such solvents can greatly enhance the sample solubility and thus the potential production rate for the separation. Immobilised columns can be used in normal- and reverse-phase modes.
In circumstances where a conventional, coated column is specified (in a validated procedure where an immobilised column is not yet approved, or when an existing method meets the specs of the separation) it may be appropriate to use coated columns rather than immobilised columns. You can contact us to inquire about our free-of-charge column screening service (see Question 6).
Method development for chiral separation using our immobilized chiral columns is streamlined by their compatibility with a wide range of solvents.
As already mentioned, immobilised polysaccharide derived CSPs have a large number of benefits that should be considered in the screening process. Based on exhaustive investigations on immobilised columns, it was concluded that a group of solvents and their mixtures can lead to better selectivity values for most of the compounds tested. It could be considered as a primary set of mobile phases for screening with these CSPs. Primary solvent configurations for the analytical screening system are composed of heptane/ethanol, heptane/2 propanol, dichloromethane (DCM) based and methyl tert butyl ether (MtBE)-based mixtures (Table 1).
Chlorinated solvents are usually submitted to a more rigorous control than the rest, due to environmental reasons. Nevertheless, their use at analytical level may be allowed in most laboratories, provided that their waste residues are treated appropriately. If the use of DCM is constrained due to environmental restrictions, tetrahydrofuran (THF)-based mixtures could be implemented instead.
If additional screening of mobile phases was required, the use of the following mixtures as a secondary set was recommended: heptane/ethyl acetate (EtOAc), heptane/ THF, acetonitrile (ACN) and pure alcohols (ethanol (EtOH) or 2 propanol (2-PrOH)) or their mixtures (Table 1).
Table 1. Mobile phases for sample screening with immobilised CSPs (CHIRALPAK IA, IB, IC, ID, IE and IF columns)
| Solvent mixture | Starting condition | Typical optimization range | |
| Alkane/EtOH | 80/20 | 99/1 to 50/50 | |
| Primary | Alkane/2-PrOH | 80/20 | 99/1 to 50/50 |
| mobile phases | Alkane/DCM/EtOH | 50/50/2 | 85/15/0 to 0/80/20 |
| Alkane/MtBE/EtOH | 0/98/2 | 80/20/0 to 0/40/60 | |
| Alkane/EtOAc | 50/50 | 80/20 | |
| Secondary | Alkane/THF | 70/30 | 95/5 to 0/100 |
| mobile phases | MeOH | 100 | |
| ACN | 100 |
Different alcohols, with shorter or longer aliphatic chains, e.g. EtOH, n-propanol and 2-PrOH, can play a “dual” role to mix either with alkanes or with acetonitrile and methanol. However, the solvent nature varies little and hence the choice stays quite limited if the viscosity factor is considered. In contrast, solvents of such as THF, EtOAc, MtBE, DCM, etc. belong to different solvent families with significantly different properties. Unlike heptane, methanol and acetonitrile, those solvents are all of mid polarity and miscible with both apolar and polar solvents. This feature is beneficial for fine-tuning composition of mobile phases, as it allows easy adjustment in retention, selectivity and resolution.
Note that DCM, THF, ethyl acetate and MTBE will destroy conventional, coated polysaccharide-based chiral columns and should only be used with the new immobilised columns.
References:
Zhang T, Franco P, Analytical and preparative potential of immobilised polysaccharide-derived chiral stationary phases, in Chiral Separation Techniques – A Practical Approach, third ed., G. Subramanian (Editor), Wiley-VCH, Weinheim, Germany, 2006, 99-134
Common approaches for efficient method development with immobilised polysaccharide-derived chiral stationary phases, P. Franco, T. Zhang, J. Chromatogr. B, 875 (2008) 48
P. Franco, T. Zhang, Common screening approaches for efficient analytical method development in LC and SFC on columns packed with immobilised polysaccharide stationary phases, in Chiral Separations, Methods and Protocols, second ed., G. Scriba (Editor), Humana Press, 2013, 113-126
Enantiomer resolution screening strategy using multiple immobilised polysaccharide-based chiral stationary phases, T. Zhang, D. Nguyen, P. Franco, J. Chromatogr. A, 1191 (2008) 214-222
Solvent versatility of immobilised 3,5-dimethylphenylcarbamate of amylose in enantiomeric separations by HPLC, T. Zhang, C. Kientzy, P. Franco, A. Ohnishi, Y. Kagamihara, H. Kurosawa, J. Chromatogr. A, 1075 (2005) 65-75
Cellulose 3,5-dimethylphenylcarbamate immobilised on silica: A new chiral stationary phase for the analysis of enantiomers, T. Zhang, D. Nguyen, P. Franco, T. Murakami, A. Ohnishi, H. Kurosawa, Anal. Chim. Acta, 557 (2006) 221-228
Cellulose tris(3,5-dichlorophenylcarbamate) immobilised on silica: A novel chiral stationary phase for resolution of enantiomers, T. Zhang, D. Nguyen, P. Franco, Y. Isobe, T. Michishita, T. Murakami, J. Pharm. Biomed. Anal., 46 (2008) 882-891
To learn more, click here.
The approach of method development and optimization on the coated CSPs can be based on a similar strategy to the one used for the immobilised-type phases. However, the choice of solvents would be restricted to those compatible with these supports and all mobile phases that could potentially dissolve the polysaccharide derivative must be avoided. The screening set will then be composed of alkane/EtOH, alkane/2-PrOH, alcohol-based and ACN-based mixtures (Table 2). In practice, these mobile phase systems are normally set up using HPLC screening systems, but often ACN or its mixtures with alcohols will be screened separately (or with accurate intermediate rinsing), in order to avoid any potential accidental mixing between alkane and ACN. If such an event happens, the coated column could be irreversibly damaged.
Table 2. Mobile phases for sample screening with coated CSPs
| Solvent mixture | Starting condition | Typical optimization range |
| Alkane / EtOH | 80/20 | 99/1 to 50/50 |
| Alkane / 2-PrOH | 80/20 | 99/1 to 50/50 |
| MeOH | 100% | |
| ACN | 100% |
It is important to point out that the conditions described in Table 1 and Table 2 for HPLC in normal phase conditions are suitable for neutral compounds. For basic and acidic samples, it may be necessary to incorporate an additive in the mobile phase in order to allow the recognition and optimize the separation. Thus, basic samples may require a basic additive (DEA, butylamine, ethanolamine, ethylenediamine…) and acidic compounds the addition of an acid (TFA, acetic or formic acid…). The percentage needed is typically 0.1% and should normally not exceed 0.5%. In SFC this amount is also respected, although as added in the co-solvent it maybe 0.5 1%, but keeping the overall concentration in the final mobile phase. In RP-conditions, buffers will be used as included in the corresponding section.
It has been found that certain amines, such as EDA (ethylenediamine) and 2 aminoethanol (AE) induce much better behaviour for certain compounds than the more commonly used DEA. Resolution degree and peak symmetry can be dramatically improved with this type of additives. For practical purposes, as EDA and AE are often not miscible in the absence of alcohol, it is better running screenings with DEA, but it will be important in the optimization step to consider this fact. However, due to their limited miscibility with certain non-polar solvents, they are only recommended when at least 2% of ethanol or methanol are present in the mobile phase.
Always consult the Instruction Manual shipped with your column, before exposing your column to any mobile phase. The immobilised chiral columns, CHIRALPAK IA, IB, IC, IE, IF and IG may be used with any solvent. This is not true for traditional Daicel coated columns, but by taking a few simple precautions, you can greatly enhance their lifetimes. The Instruction Manuals refers to solvents that can be used with that specific column. You should carefully heed the caution statements found at the top of each Instruction Manuals for solvents to avoid. Some solvents listed may be acceptable as mixtures. Mixtures of three solvents should be avoided on the coated columns as the solubility of the polysaccharide polymer is unknown and may be increased. This should not be a problem on the immobilised columns, although for a good chromatographic performance the miscibility of the solvents involved in the mobile phase should be always guaranteed.
In contrast to immobilised columns, traditional Daicel coated columns should never be used with solvents such as methylene chloride, chloroform, THF and DMSO. Such solvents solubilize the polysaccharide polymer at the head of the column which then reprecipitates as the solvent is diluted, resulting in a plugged column. Please be aware that even small quantities of incompatible solvents introduced in sample dilutions, or left in transfer lines (including autosampler lines) can rapidly degrade or destroy a column. Even residual amounts of forbidden solvents in samples may shorten the life of the column. For these reasons, we recommend using the immobilised columns.
If you are unsure whether or not a particular solvent can be used with your column, assume that it is incompatible and avoid using it until you have contacted us. Depending on your region, please contact us: Chiral Technologies Europe (CTE), support@cte.daicel.com, Chiral Technologies, Inc. (CTI), questions@cti.daicel.com, or Daicel Chiral Technologies India (DCTI),chiral@chiral.daicel.com
Replacement Instruction Manuals can be found under Quick Links on any page of the website. Or they can be sent to you by FAX or e-mail; contact us as suggested above if you need a replacement.
Several of our chiral columns, including the immobilized series, are compatible with reverse-phase chromatography for chiral separation.
The polysaccharide type CSPs seem to be most frequently used with organic eluents (alkane mixtures or the polar solvents discussed above). However, resolution of enantiomers on these columns by aqueous eluents has a history almost as long as their applications with organic mobile phases [K. Tachibana et al., J. Chromatogr. A, 906 (2001) 127]. The choice of RP mode has often been related to the need for direct injection of the samples of biological extracts; to address the low retentivity of certain compounds under normal phase conditions, the low solubility of polar and ionic samples in organic mobile phases or the lack of appropriate enantioselectivity in organic solvents. An additional and increasingly important reason to favorite RP methods is their suitability for LC/MS applications.
RP chromatography is often complementary to normal phase (NP) mobile phases. The presence of water in the mobile phase undoubtedly has an impact on the enantiorecognition and offers the possibility of enhancing the chances of resolution success.
The nature of the molecules to be analysed should be taken into account for mobile phase selection. Under this primary guideline, a “decision tree” can be defined to start the method development. To learn more, click here.
The selected mobile phases do not really differ from the proposals for achiral RP screening.
The selection of the mobile phase for neutral compounds is straightforward: plain water is suitable to be used as the aqueous component. As for the additives, the most common and effective pH value of the aqueous solution for the resolution of acidic molecules is in the range 2.0-2.5 in which the ionisation of most organic acids will be suppressed. Formic acid is a good choice where LC/MS compatibility is required while other acids (TFA, acetic acid, H3PO4) may be used for other applications. Where RP methods suitable for MS detection for basic compounds are being developed, ammonium bicarbonate adjusted to pH 9 is an excellent option, although some other buffers or solutions at basic pH (phosphates, borates, ammonium acetate or KPF6 aqueous solutions) may also be useful in terms of enantiorecognition.
The organic modifier plays an important role in regulating retention and modulating enantioselectivity. The first choices of modifiers to be combined with water are acetonitrile (ACN) and methanol (MeOH). The use of other organic modifiers, such as EtOH, 1-PrOH and 2-PrOH can also be considered. In our practice, however, these modifiers would be tried only if no successful hit is achieved with ACN and MeOH.
This is the typical pattern for method development in RP-mode with any of our polysaccharide-derived columns. On the immobilised supports, other solvents, such as THF, can be employed. Moreover, sample injection can also be performed in DMSO, DMF or acetone, for example, in case of need. THF may sometimes be a good alternative to ACN and MeOH, but in a general way, it seems to be a less versatile organic modifier if compared with ACN and MeOH. The relative eluting strength of these three organic modifiers can be ranked as follows: THF > ACN > MeOH.
An interesting feature of immobilised polysaccharide-derived supports is that it would be possible to use the same column in organic and water containing solvents without compromising the reproducibility of results on both modes. This possibility does exist due to the fact that a washing protocol between RP and organic mode in strong solvents (such as THF and DMF) can be applied, in order to eliminate water traces adsorbed on the polymer. This protocol is described in detail on the instruction manual of the immobilised columns, but will not be compatible with the previously existing coated CSPs.
To learn more about the preparation of buffers, including also the MS-compatible ones, click here.
References for RP-method development:
Reversed-phase screening strategies for liquid chromatography on polysaccharide-derived chiral stationary phases, T. Zhang, D. Nguyen, P. Franco, J. Chromatogr. A, 1217 (2010) 1048–1055
P. Franco, T. Zhang, Common screening approaches for efficient analytical method development in LC and SFC on columns packed with immobilised polysaccharide stationary phases, in Chiral Separations, Methods and Protocols, second ed., G. Scriba (Editor), Humana Press, 2013, 113-126
Reversed-phase liquid chromatographic separation of enantiomers on polysaccharide type chiral stationary phases, K. Tachibana, A. Ohnishi, J. Chromatogr. A, 906 (2001) 127-154
SFC applications in the separation of enantiomers are a growing field and a number of applications have been already described. Experiments to date with polysaccharide-derived CSPs show that excellent success rates can be achieved by using alcohol co-solvents. In particular, MeOH, EtOH, and 2-PrOH are very good initial co-solvent choices. ACN seems to be less successful, but it may be an option for a secondary screening. If the elution of the analytes is not sufficient when ACN is used, an alcohol can be added to the CO2/ACN mixture. To learn more about method development strategies, click here.
To resolve basic compounds, add a small amount (normally 0.5-1% in the co-solvent) of a basic additive (often DEA or TEA). For acidic compounds, an acidic additive (i.e. TFA, formic or acetic acid) may be helpful for better resolution, although the acidity of CO2 could in certain cases be sufficient for good enantiomer resolution acidic compounds.
Moreover, immobilised polysaccharide-derived CSPs are compatible with a much wider range of solvents than are the coated phases. The use of such “extended range” solvents was investigated for SFC screening in a similar manner as for LC separations. Initially these solvents were used to enhance sample solubility, to solve difficult separations or to process compounds being unstable in alcohols. Subsequently we have found that these solvents can also offer exceptional enantioselectivity profiles. THF, MtBE, DCM or ethyl acetate, for example, can be used as the CO2 modifier. In some cases, pure DCM, MtBE and ethyl acetate may not be strong enough to elute certain compounds. In these cases, the addition of small percentages of an alcohol mixed with the co-solvent is advised. To learn more about method development strategies, click here.
As already mentioned, success rate is already very high with the alcohol modifiers. Therefore, it has become established practice to use either the immobilised columns or alternatively the coated ones – in the column set for screening with the alcohol modifiers as first strategy. Then the solvent range can be broadened on the immobilised columns if no satisfactory separation is achieved or sample solubility/stability becomes an issue. SFC and LC are complementary techniques and comparable success rates are achieved with the same set of columns.
Analytes are preferably dissolved in the solvent mixture that is used as the co-solvent in SFC. If this is not possible, choose a better sample diluent (e.g., ethanol) and monitor any potential perturbance of the baseline due to the injection solvent. For samples insoluble in alcohols or ACN, it is possible to dissolve these in DCM or DCM mixtures for injection when using immobilised phases. In such cases, caution should be taken to avoid on-line precipitation of the compounds. This step cannot be done with coated phases without compromising the life-time of the column. Please note that coated CSPs are only compatible with alcohols and acetonitrile mixtures in SFC.
A common concern is the effects of high pressures used in SFC on column stability. The pressure drop across the column is the important factor in column stability. This pressure drop is lower in SFC than HPLC and Daicel columns have proved very stable to SFC conditions. We recommend that when the column is not in use, it be removed from the SFC, flushed briefly with isopropanol to displace CO2 (that would evaporate leaving a dry column), and capped. When using a Daicel column in SFC that had been used in HPLC, it is necessary to first flush the hexane with isopropanol, as CO2 will not efficiently flush hexane, and a noisy baseline will result. Chiral Technologies has recently extended its line of Daicel SFC columns to new dimensions: 3-mm internal diameter and different column lengths. These new columns, packed with 3-µm immobilised or coated CSPs, are designed for ultra-fast SFC analysis. To learn more about high-speed and efficient chiral separations, in less than one minute, using the 3-mm chiral SFC columns, click here.
SFC can offer several advantages in preparative applications. Separations are faster and isolation of the product from the mobile phase is also faster as the bulk of the mobile phase evaporates as part of the collection process. With the lower pressure drop experienced in SFC, the use of higher efficiency 5-micron columns for preparative application is feasible. Chiral Technologies offers 1-, 2-, 3- and 5-cm i.d. columns packed with 5 µm materials in SFC column hardware. For analytical screening 3-micron columns are also available. Our technical teams will help you chose the best column dimensions commensurate with SFC systems available in your lab. Depending on your region, please contact us: Chiral Technologies Europe (CTE), support@cte.daicel.com, Chiral Technologies, Inc. (CTI), questions@cti.daicel.com, or Daicel Chiral Technologies India (DCTI), chiral@chiral.daicel.com
References for SFC-method development:
P. Franco, T. Zhang, Common screening approaches for efficient analytical method development in LC and SFC on columns packed with immobilised polysaccharide stationary phases, in Chiral Separations, Methods and Protocols, second ed., G. Scriba (Editor), Humana Press, 2013, 113-126
Enantiomer resolution screening strategy using multiple immobilised polysaccharide-based chiral stationary phases, T. Zhang, D. Nguyen, P. Franco, J. Chromatogr. A, 1191 (2008) 214-222
Separation of enantiomers and conformers of Tofisopam using Daicel immobilised polysaccharide-derived chiral columns using the Agilent 1260 Infinity Analytical SFC System, T. Zhang, N. Nguyen, P. Franco, M. Vollmer, Application Note (Agilent , 2011)
Enantiomer separation of non-steroidal anti-inflammatory drugs, T. Zhang, N. Nguyen, P. Franco, M. Vollmer, Application Note (Agilent, 2011)
The use of gradients could also be envisaged to select appropriate solvent composition in terms of retention and selectivity in HPLC and SFC modes. Daicel chiral columns are perfectly compatible with them. However, as two enantiomers do not have a different polarity, it would make much sense to set the final analytical method under isocratic conditions.
When setting up a gradient screening, please keep in mind the following points:
- The time required to re-equilibrate the column back to initial mobile phase conditions. Depending on the number of samples, it would be important to evaluate whether there are any time-savings realized from the use of a gradient.
- It would then be important to make sure especially in HPLC-mode that the chromatographic system is able to guarantee proper/accurate metering of mobile phase composition with organic solvents, as this is not always the case with DCM, EtOAc or THF mixtures. Please note that most HPLC systems are designed to deliver good gradients in RP-mode, but they do not work properly in normal phase conditions.
The pressure limit specified in the Instruction Manual for a Daicel chiral column applies only to the pressure drop across the column itself and not to the rest of the chromatographic system. For example, if the total pressure drop measurement in your system, at normal operating conditions, is 1000 psi, but the system pressure (without column) is 300 psi, then the pressure drop across the column would be 700 psi, which would be acceptable for most columns. A high system pressure probably indicates that there is a partial blockage, possibly in connecting tubing, an in-line filter, or a valve channel. Whenever possible, this blockage should be systematically located and the problem component replaced.
High operating pressures often result from material blocking the frits of the column. In some cases, this material may be removed through the use of recommended washing procedures. To prevent such problems, it is always wise to use a replaceable in-line filter or guard column before the analytical or semi-preparative column.
Flow rates for semi-preparative columns can generally be scaled up from the flow rate developed on an analytical column, by a factor proportional to the volume comparison of the two columns. When operating at the higher semi-preparative flow rate, you may need to increase the diameter of connecting tubing or the volume of the detector flow cell in your system. Alternatively, you may need to slightly reduce the flow rate on the semi-preparative column to stay under the recommended maximum operating pressure.
High back-pressures may result if a column is eluted at normal flow rates with solvents such as pure ethanol or isopropanol, due to the high viscosity of these solvents. If you are experiencing high back-pressure when using these solvents, reduce the flow rate to a level that brings the back-pressure under the recommended maximum operating pressure. Since these solvents are often associated with column-cleaning procedures, you should be able to operate at higher flow rates once you return to your normal operating mobile phase.
A rapid build-up in pressure with the traditional coated columns can be a symptom of a serious column problem. Introduction of incompatible materials (from sample or mobile phase) can be rapidly destructive to a column. When such a problem occurs, chiral stationary phase will dissolve or be lifted into the mobile phase, only to drop out of solution downstream, plugging the flow path, and causing a rapid build-up of column pressure.
Column performance can be measured by many parameters. These include column efficiency, selectivity and resolution, peak symmetry and column pressure drop. There are many possible reasons for the change of column performance while in operation. Some are the normal chromatographic problems which can occur with any column. Some, especially for coated Daicel columns, relate to specific properties of the columns and of the stationary phases.
In general, if a column problem is suspected, it should first be thoroughly flushed (see its operating instructions) and then tested under the QC conditions used when it was originally packed. The results of this test can usually help diagnose the problem.
Since the solution of such problems is simpler when immobilised columns—CHIRALPAK IA, IB, IC, ID, IE, IF and IG are used, the resolution of problems with these columns will be addressed first. Later we will note differences between the traditional coated columns and the immobilised columns.
Operating Pressure: With immobilised columns, the source of an increase in operating pressure is usually the inlet frit. This can be blocked either by solids in the sample or entrained in the mobile phase. They can also be blocked by the introduction of a sample which was dissolved in a solvent stronger than the mobile phase; as it mixes with the mobile phase, material can be precipitated from solution and is filtered out by the frit. This can be corrected by changing or cleaning the inlet frit. It is sometimes difficult to remove the inlet frit and such removal always comes with the danger of disturbing the packed bed of the column. One easy experiment is to reverse the flow direction through the column in the hope that the foreign matter will be washed from the frit. It is, of course, always better to prevent such problems by the use of (and regular replacement of) a guard cartridge.
Sudden increases in operating pressure with traditional coated columns can be due to the effects of solvents on the chiral stationary phase or to sample solubility issues. If the pressure increase is due to the introduction of a solvent which can damage the stationary phase, it is usually too late. To prevent such an occurrence, it is vital to ensure that the entire HPLC system is flushed of potentially harmful solvents before the column is connected to the system. Proper sample clean-up and preparation are also vital. Small amounts of non-allowed solvent in a sample preparation may seem insignificant, but these low-level residues often dissolve the chiral polymer, which leads to a rapid decay in column performance. In these cases, the test chromatogram will almost certainly show a marked drop in column efficiency and selectivity. Although in some cases prolonged flushing with 2-propanol may improve the situation, usually the column is most likely dead and will need to be replaced.
Column Efficiency: In most cases, changes in column efficiency are accompanied by changes in peak symmetry or peak shape. In rare cases, a reduction in efficiency accompanied by the appearance of shoulders on the peak trailing edge may be due to void formation at the head of the column. This could be due to dissolution of the silica support by the mobile phase conditions (usually in reverse-phase mode), over-pressurizing of the column, collapsing the silica particles or packing disturbances in the column. Most loss in efficiency problems are due either to partial blockage of the inlet frit (see above) or are due to the adsorption of material at the head of the column.
Adsorption of material at the head of the column can be seen where the samples are not pure and contain components which are strongly adsorbed on the stationary phase. This can often be resolved when using immobilised columns by flushing with a strong solvent such as THF or DMF (please refer to instruction manuals for detailed protocols). This approach cannot be realized with the coated columns and the best that can be done is to flush them with the strongest compatible solvent, often 2-propanol. For those cases in which recommended washing fails to restore performance, more drastic washing may be needed. Such washing procedures carry a significant risk of column damage, so they are best used as a measure of last resort.
For more information on such procedures, contact us: Chiral Technologies Europe (CTE), support@cte.daicel.com, Chiral Technologies, Inc. (CTI), questions@cti.daicel.com, or Daicel Chiral Technologies India (DCTI), chiral@chiral.daicel.com
This approach is rarely successful and represents another reason why we strongly recommend using the immobilised columns wherever possible.
Sometimes an established separation cannot be duplicated on a new column. We carefully ensure minimum lot-to-lot variation in column performance and this situation more often results because the established separation is dependent on some type of column conditioning that the new column has yet to be subjected to. The older column may have a “memory effect” in which additives used in the past history of the column have become adsorbed on the stationary phase, and are crucial to the current separation. With immobilised columns, a simple flush with DMF (followed by EtOH rinsing, as described in regeneration protocol) may be all that is necessary to “reset” the stationary phase. In many cases, the problem can be resolved by conditioning the new column for a few hours with mobile phase that contains the pertinent additive. In those cases in which the separation is still not restored, the method may need to be redeveloped with a different mobile phase, column, or temperature. For this reason, we recommended developing new separations on a new column, or one for which the mobile phase and sample history are documented. Validated methods should be established with more than one column.
E-mail questions concerning columns which are not working properly are always welcome. Contact us: Chiral Technologies Europe (CTE), support@cte.daicel.com, Chiral Technologies, Inc. (CTI), questions@cti.daicel.com, or Daicel Chiral Technologies India (DCTI), chiral@chiral.daicel.com
To avoid delay in receiving an accurate response to your question, describe your problem as completely as possible.
Our Chiral Separation Service is an important part of the comprehensive offering of products and services we provide. This service is available to clients who need to quickly obtain pure enantiomers (or isomers), but who don’t have the time, equipment, range of columns, or facility to scale-up an analytical separation.
To initiate a Separation Service project please contact us: Chiral Technologies Europe (CTE), support@cte.daicel.com, Chiral Technologies USA (CTI), separations@cti.daicel.com,or Daicel Chiral Technologies India (DCTI), chiral@chiral.daicel.com
If required we can review your confidentiality agreement. Please provide us with 50 mg of racemate for a small scale separation or 250 to 300 mg samples for the evaluation of separations of 1 kg or above. Our expert staff then use the wide range of commercial Daicel CSPs to identify the optimum conditions for a preparative separation of your compound. When a promising separation is identified, you will then be provided with a quotation for the separation to be performed. Enantiomers are typically returned with an optical purity of >98% e.e. (chemical purity = purity of the racemate and yield >85% for each isomer). Timescale for screening to quotation for small scale separations is 2 to 3 days and for larger scale separations is 2 weeks.
Analytical separations can be predictably scaled up to the semi-preparative level using some fairly simple calculations. When resolution of a given quantity of racemate is the desired objective, it is best to first optimize using an analytical column to yield the maximum possible loading. If you are fortunate to have more than one possible separation method, each method can be tested separately to determine which one gives the best overall loading. For each attempt to achieve maximum loading, start by making injections of a solution of the target racemate, as concentrated as possible, in the mobile phase. Using a detection wavelength selected to keep the peaks on scale, increase the injection volume until the valley between the enantiomers begins to rise.
This should give you an experimental loading weight (WE): WE = Cmax x VAmax
where Cmaxis the maximum concentration of racemate and VAmax is the largest analytical injection volume before overload.
For ballpark estimation purposes, typical WE values for an analytical column (0.46 x 25 cm) are 1 10 mg/injection.
The relative loading capacity (LCR) on the 0.46 x 25 cm analytical column is assumed to be “1.” For various-size semi-preparative and preparative columns, the relative loading capacity and associated flow rates can be determined from the following table.
| Column Size (i.d. x Length) | Weight of Packing (g) | Loading Capacity (relative) LCR | Flow Rate (ml/min.) |
| 0.46 x 25 cm | 2.50 | 1 | 1.0 |
| 1 x 25 cm | 11.8 | 5 | 5.0 |
| 2 x 25 cm | 47.4 | 19 | 19 |
| 5 x 50 cm | 592 | 250 | 50 |
| 10 x 50 cm | 2370 | 1000 | 200 |
How much can I load?
The loading on the preparative column (WPREP) would be calculated with the experimental weight on the analytical column (WE) and the relative loading capacity (LCR).
WPREP = WE x LCR
Thus, if the typical load on an analytical column (0.46 x 25 cm) is 1-10 mg, then a typical load on a 2 x 25 cm semi-preparative column is 19 –190 mg/injection.
What size column do I need? This is a typical preparative question that is inter-related to the question: How many injections (N) are you willing to make? To determine the size column that you need, the following equation can be used:
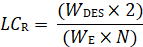
where LCR is the loading capacity of the column needed to achieve your objectives, WDES is the weight of desired enantiomer you want to separate and WE is the experimental weight on the analytical column.
EXAMPLE: A researcher needs to isolate 1 gram each of two enantiomers from a racemic mixture (WDES). It has been determined that 48 half-hour runs (N) made in a 24-hour period would be a reasonable number of preparative injections. The maximum analytical loading weight is 2 mg = .002 g (1 mg of each isomer, WE). What relative loading capacity is required if 48 injections are to be made?
Consulting the table, it can be seen that a 2 x 25 cm column with a relative loading capacity of 19 would probably best meet the researcher’s needs.
The older CHIRALCEL and CHIRALPAK columns are based on 10-micron particles, whereas H-series CHIRALPAK and CHIRALCEL columns are based on 5-micron particles. The H-series columns have chiral stationary phases identical to their non-H counterparts (i.e., an AD and AD-H column have the same chiral stationary phase), which provide similar selectivity to the standard columns while providing much better chromatographic efficiency. The smaller 5-micron particles thus give better overall resolution. This means better performance for the most difficult separations, or for separations in which impurities would otherwise interfere with the main components. For those situations in which speed of analysis is most important, a 15 cm H-series column may give an equivalent separation with a shorter analysis time, than the same separation on a 25 cm non-H column. -RH series (reverse phase) columns are also based upon 5-micron particles.
In this context, it can be noted that the immobilised columns, CHIRALPAK IA, IB, IC, ID, IE and IF are available in 10-, 5- and 3-micron particle sizes for analytical separations.
Part A – Things To Do when Using Daicel Chiral Columns
- Carefully read the Instruction Manual. There are major differences in stability between the immobilised columns and the traditional coated Daicel columns, as well as between individual coated columns.
- Flush the entire HPLC system with the appropriate solvent (including the sample loop, autosampler rinse solvent [if used], and detector), before attaching the column to the instrument.
- Use only recommended solvents to ensure maximum column life. A list of alternative solvents is available from Chiral Technologies. Please note, however, that not all of the alternative solvents have been evaluated overall mixture ranges and have not been evaluated over extended periods of use. Consequently, prolonged use of these alternative solvents could shorten column life considerably.
- Use simple mobile phases. Chromatographic separations on normal-phase columns are usually achieved with simple mobile phases such as heptane/isopropyl alcohol (IPA), 95/5 to 50/50 v/v, or heptane/ethanol (EtOH), 95/5 to 50/50 v/v. Note: several of the older polysaccharide columns are not stable to alcohol percentages over 15% (see individual column Instruction Manuals that are provided with each column for solvent limitations). HPLC-grade EtOH is used for methods developed at Chiral Technologies, but you should be aware that sometimes HPLC-grade EtOH is denatured with 5% IPA and 5% MeOH. This can obviously have an influence in the resolution. DO NOT USE EtOH DENATURED WITH BENZENE OR OTHER NON-ALCOHOL DENATURANTS.
- Equilibrate the system to a stable baseline after attaching the column and starting the solvent flow. Equilibration usually requires a minimum of thirty minutes at a flow rate of 1 ml/min. At lower flow rates or lower detector sensitivity, longer equilibration times may be required.
- Samples should be free of insoluble particulates. It is recommended that a guard column always be used to prevent contamination of the main column. Note: Guard columns are available for all the polysaccharide chiral phases. It has been our experience that a significant number of column problems arise due to the plugging of column frits. This problem can be completely eliminated by using a guard column or an inline filter (2 micron or less).
- To distinguish enantiomers from achiral impurities, try running at multiple wavelengths (chiral peaks will have the same relative proportion to each other at all wavelengths), or use different types of detectors such as a chiral detector and/or a refractive index detector. For racemic compounds with multiple stereogenic centers (chiral centers), each pair of enantiomers will retain the same signal ratio at all wavelengths.
- Dissolve the sample in mobile phase constituents only, to avoid possible on-column precipitation and/or injected solvent effects. If the mobile phase will not dissolve the sample, contact Chiral Technologies for assistance.
- Flush the column with the appropriate storage solvent when the analysis is completed. Aqueous buffers are commonly used as a mobile phase component when using columns in the reversed-phase mode. When a buffer solution has been used, it is imperative that the column be flushed with the identical mobile phase, without the buffering salt present, before the column is converted to the recommended storage solvent. In addition, when mobile phase modifiers (i.e., acids or bases) are used, the columns should be flushed thoroughly with the same mobile phase, without the modifier present, before flushing the column with storage solvent. When acidic or basic modifiers, such as trifluoroacetic acid (TFA) or N,N-diethyamine (DEA), are used as mobile phase modifiers, it is satisfactory to leave this mobile phase in the column overnight. However, if the column will not be used for several days it is recommended that the system be flushed with a mobile phase that does not contain modifiers so that the column is not damaged. Note: When the column is no longer being used, it should be removed from the HPLC system and capped tightly at both ends to avoid evaporation of the solvent. When these polysaccharide columns are used in the normal-phase mode without the modifiers, the column can remain attached to the HPLC system for up to a week without being flushed. Polysaccharide columns last for years under proper care but can degrade quickly if the storage instructions are not followed.
Part B – Things Not To Do When Using Daicel Chiral Columns
- Do not operate your Daicel column above the recommended maximum pressure limit.
- Do not use dilution solvents and mobile phases that are not listed on the Instruction Sheet for your column. Not all columns are compatible with the same solvents; therefore, don’t assume that a special solvent that worked fine on a different column previously will be OK on your current column.
- Do not leave buffers and additives in your column if you are planning to store it for a long time. Do follow the recommended storage instructions that are found on the Instruction Sheet that comes with each column.
- Do not discard the test chromatogram that comes with each column. The Instruction Manual for your column can be found under Quick Links on any page of the website. Or contact us: Chiral Technologies Europe (CTE), support@cte.daicel.com, Chiral Technologies, Inc. (CTI), questions@cti.daicel.com, or Daicel Chiral Technologies India (DCTI), chiral@chiral.daicel.com
If your column develops a performance problem, it may be necessary to test it to determine whether or not it has the same selectivity and efficiency as it had when new. Having the original test chromatogram is a good way to compare current performance to when it was new.
In many cases, installing a guard column upstream of your analytical or semi-preparative column is a cost effective strategy. The purpose of a guard column is to protect the analytical or semi-preparative column from materials that would either adsorb on the column, or which would dissolve some of the column packing. Guard columns thus serve a sacrificial function; when a guard column is nearing the failure or breakthrough point, it can be discarded and replaced at a fraction of the cost of a new column. Knowing when to replace a guard column can be determined from observations about your chromatography. Loss in separation between peak maxima, increased peak broadening or tailing, or increased pressure drop in your system are all signals that a guard column may need to be replaced.
A guard column should contain the same packing as the analytical or semi-preparative column, and specific guard columns are available for all Daicel chiral columns. A guard column containing a different chiral stationary phase may actually diminish the separation. A non-specific guard column might absorb some sample or mobile phase impurities; however, it would degrade the separation by adding more volume to the sample flow path without increasing the separation.
The end fittings for a guard column are exactly the same as the main column. Therefore a short piece of narrow i.d. connecting tubing is needed between the guard column and main column. Guard columns for all H-series and R- or RH-series columns are cartridge type. A universal cartridge holder can be used for most of our analytical 5- and 3-micron column, together with a specific disposable cartridge containing the same packing as the main column. For small-diameter columns based on 3-micron materials, on-line frits should be added instead, in order to avoid deleterious increase of system volumes. For advice, please contact us: Chiral Technologies Europe (CTE), support@cte.daicel.com, Chiral Technologies, Inc. (CTI), questions@cti.daicel.com, or Daicel Chiral Technologies India (DCTI), chiral@chiral.daicel.com
Although quite rare, it is not unknown for polysaccharide-based chiral columns, especially after repeated use of additives or aggressive mobile phase conditions, to experience some loss of performance. Fortunately these effects can be mitigated by utilizing a regeneration procedure. These regeneration procedures utilize extended range solvents like DCM, DMF, or EtOAc to help restore the columns original performance. It is important to note these procedures can only be utilized with the immobilised CHIRALPAK® chiral columns, and will result in irreversible damage to any coated CHIRALPAK® or coated CHIRALCEL® column.
For a detailed regeneration procedure for all sub-2 µm, 3 µm, and 5 µm immobilised CHIRALPAK® analytical and preparative columns, click here.
Chiral selectors are molecules or moieties that interact differently with enantiomers, enabling their separation. Our chiral columns utilize various chiral selectors, including polysaccharide chiral selectors, to achieve efficient chiral separation.
Preparative chromatography involves scaling up analytical chiral separation to isolate larger quantities of pure enantiomers. We offer a range of chiral columns and services to support preparative chromatography applications.
Chiral separation techniques encompass various chromatographic methods, including HPLC, SFC, and TLC, each employing different chiral selectors and chiral columns to achieve separation.